They say if you want to break bad news, do it on a Friday. The Food and Drug Administration did just that in releasing its final approval for genetically modified salmon to be grown and sold in the United States on Friday, March 8.
Days before resigning from his post as commissioner of the FDA, Scott Gottlieb cleared the path for the sale of Frankenfish by declaring that the FDA had no "authority to issue labeling guidance."
Despite a long, hard-fought effort first to keep this unproven product out of the U.S. market until it is shown to be safe for consumption and second to label it clearly and accurately to give consumers a window of confidence in our food system, the FDA's approval guidelines have left a wide berth for unknown risks in the consumption of a lab-created protein, and federal labeling guidelines seem almost scripted to create confusion in the marketplace.
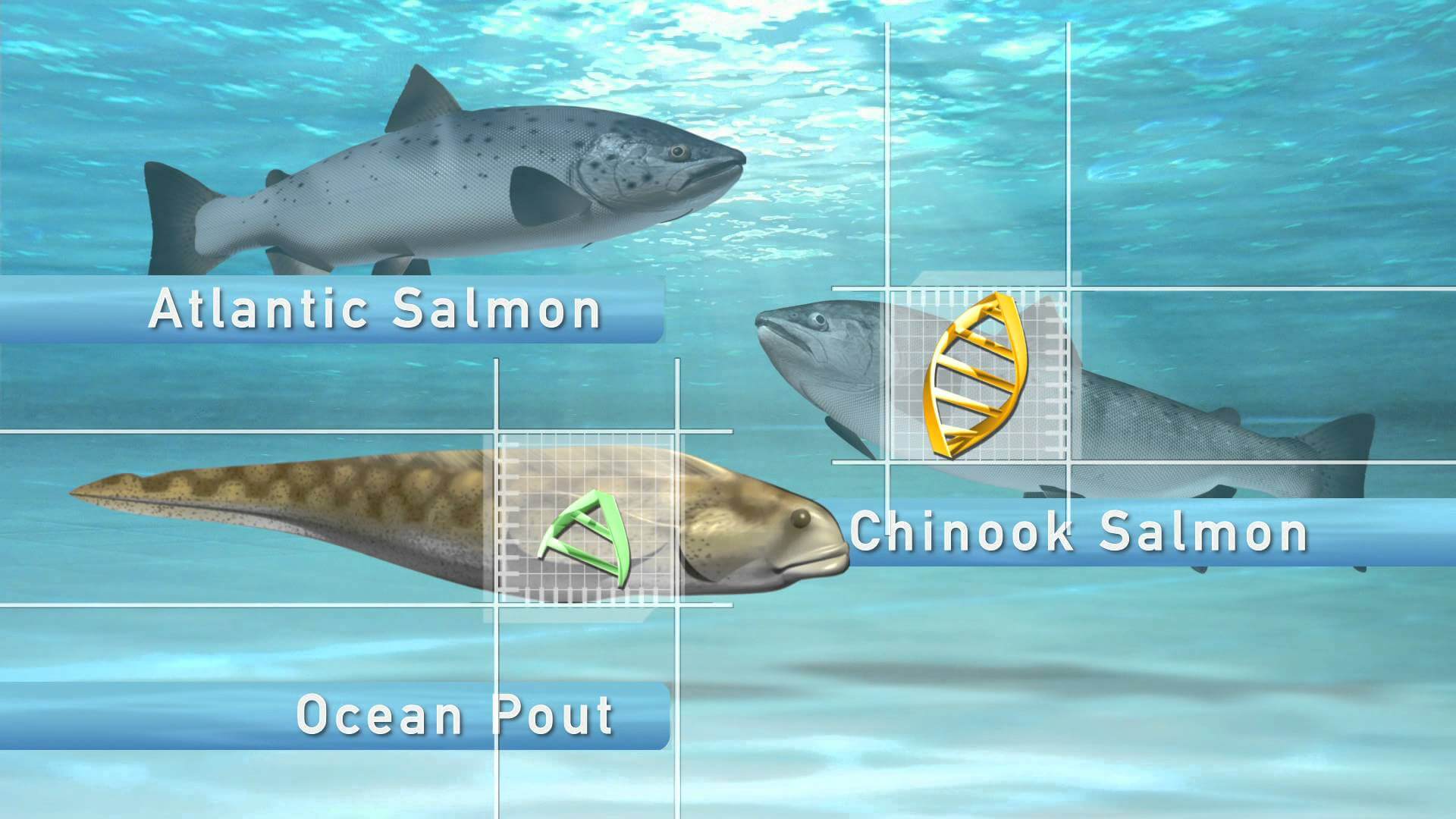
Proponents will argue that AquaBounty Technologies, creator of AquAdvantage salmon, passed all standards established by the FDA to show that their gene-spliced salmon/ocean pout hybrid is safe for consumption. The problem is that FDA standards are hardly rigorous.
AquaBounty Technologies was required to perform its own tests to establish proof of safety, per federal guidelines:
“The data in the application are provided by the company, because the burden of proof is on the sponsor, or company, to demonstrate safety and to validate the claim that is being made. As part of the review process, whenever the FDA had additional questions, the sponsor provided additional data or information.”
In other words, if you can generate data that shows your product is safe (in theory, of course), you're good to go. If you think this sounds like a reasonable standard, I recommend you look into the origins of our opioid crisis, which is founded in the same rigorous standards for proof of safety. In the case of oxycontin, the FDA approved the drug's label language that said delayed absorption "is believed to reduce the abuse liability of a drug" and that addiction to opioids "legitimately used in the management of pain is very rare." Purdue Pharma was then free to tout the drug as surprisingly UNaddictive to the nation's doctors.
We have no way of knowing whether Frankenfish will be safe for consumption over long periods of time or in what amounts, because those things are neither verifiable nor are they part of the process of approval. The first product is expected to hit U.S. markets late next year.
The last hurdle in AquaBounty's access to the U.S. market was establishing federal labeling guidelines. Clear labeling would send the message to consumers that they could feel confident that the salmon they were buying would be labeled clearly as wild, farmed Atlantic or farmed GMO.
Then in 2016, Congress passed a law mandating the Department of Agriculture to set a national standard for disclosing all bioengineered foods. Once Frankenfish was wrapped up in the same disclosures as bioengineered corn, wheat and soy, whose lobby is incredibly powerful, the hope for a clear label on GMO salmon was all but lost.
Manufacturers will be required to label GMO salmon using "text, symbol, electronic or digital link, and/or text message, with additional options available to small food manufacturers or for small or very small packages."
Unfortunately, labeling will not be required for food served in a restaurant, "cafeteria, lunch room, food stand, saloon, tavern, bar, lounge or… salad bars, delicatessens, and other food enterprises located within retail establishments that provide ready-to-eat foods."
As consumers, we should all be more than a little concerned that this newfangled fish is poised to slip into our food system virtually unannounced through the same approval process as pharmaceuticals. I guess this brings new meaning to the notion that food is medicine. The question remains: Is it good medicine or bad?